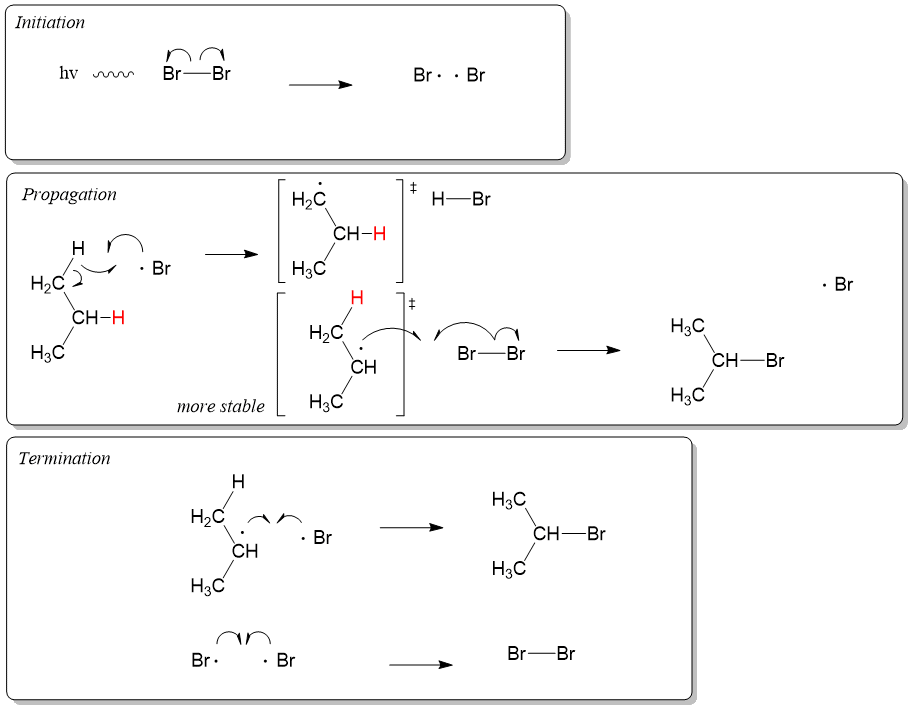
Organic Chemistry Mechanisms
The general rate law for a unimolecular elementary reaction (A → products) is. rate = k[A]. r a t e = k [ A]. For bimolecular reactions, the reaction rate depends on the number of collisions per unit time, which is proportional to the product of the concentrations of the reactants, as shown in Figur e 14.6.1 14.6. 1.
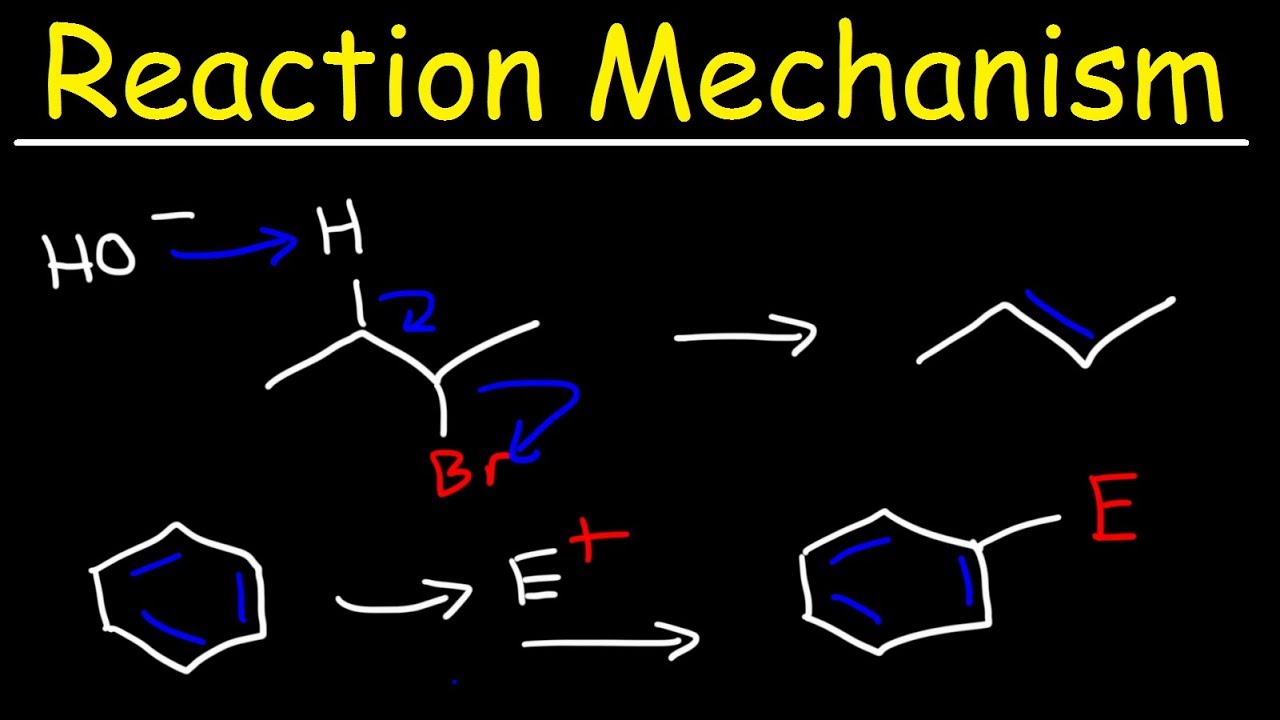
Organic Chemistry Reaction Mechanisms Addition, Elimination
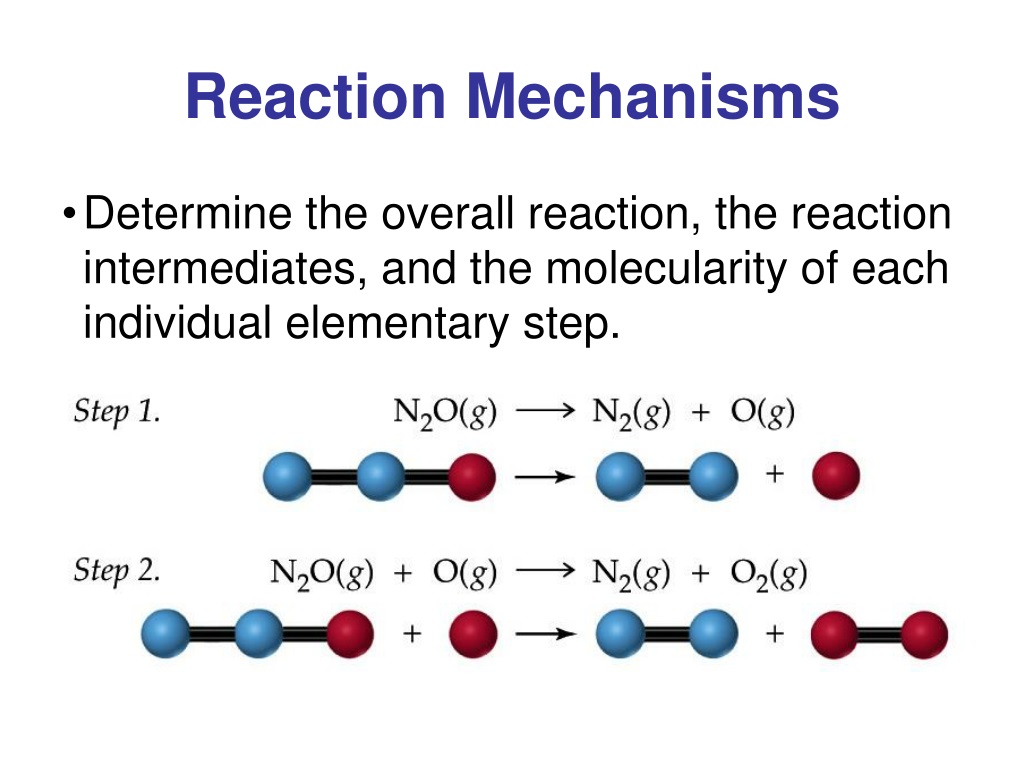
We call each step in a reaction mechanism an elementary reaction. Elementary reactions occur exactly as they are written and cannot be broken down into simpler steps. Elementary reactions add up to the overall reaction, which, for the decomposition, is: 2O3(g) 3O2(g) (4.5.2) (4.5.2) 2 O 3 ( g) 3 O 2 ( g) Notice that the oxygen atom produced in.

Reaction Mechanisms Overview ( Video ) Chemistry CK12 Foundation
This category includes two basic mechanisms used in chemistry to explain changes in material systems that conserve (e.g., phase change) or modify (e.g., chemical reaction) the chemical identity of the substances involved. The argumentative structure of each of these mechanisms is summarized in Table 1. These mechanisms are typically introduced.
PPT Reaction Mechanisms PowerPoint Presentation, free download ID
RMG is an automatic chemical reaction mechanism generator that constructs kinetic models composed of elementary chemical reaction steps using a general understanding of how molecules react. Flux diagram for the pyrolysis of 1,3-hexadiene, an example model generated with RMG, showing the net carbon flux at an instant near the end of the.
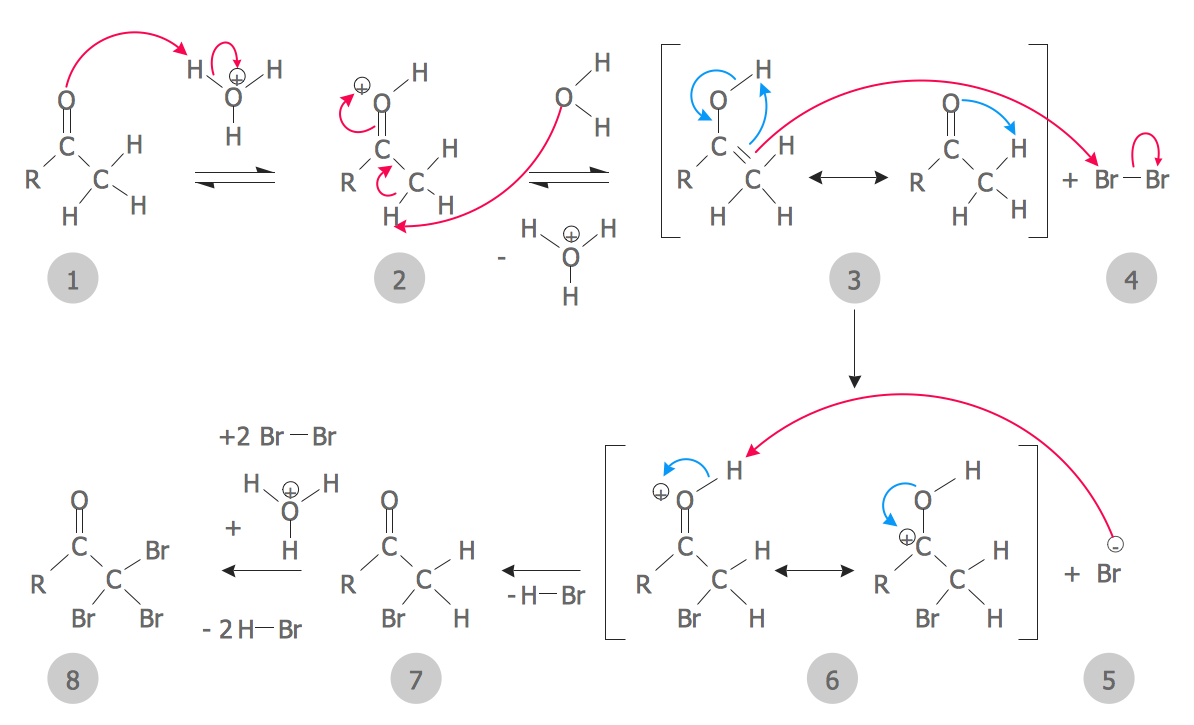
Chemistry Equation Symbols
Enhance Your Shopping Experience With Our Personalised Recommendations. Choose From a Wide Selection Of Informative and Comprehensive Books For You.
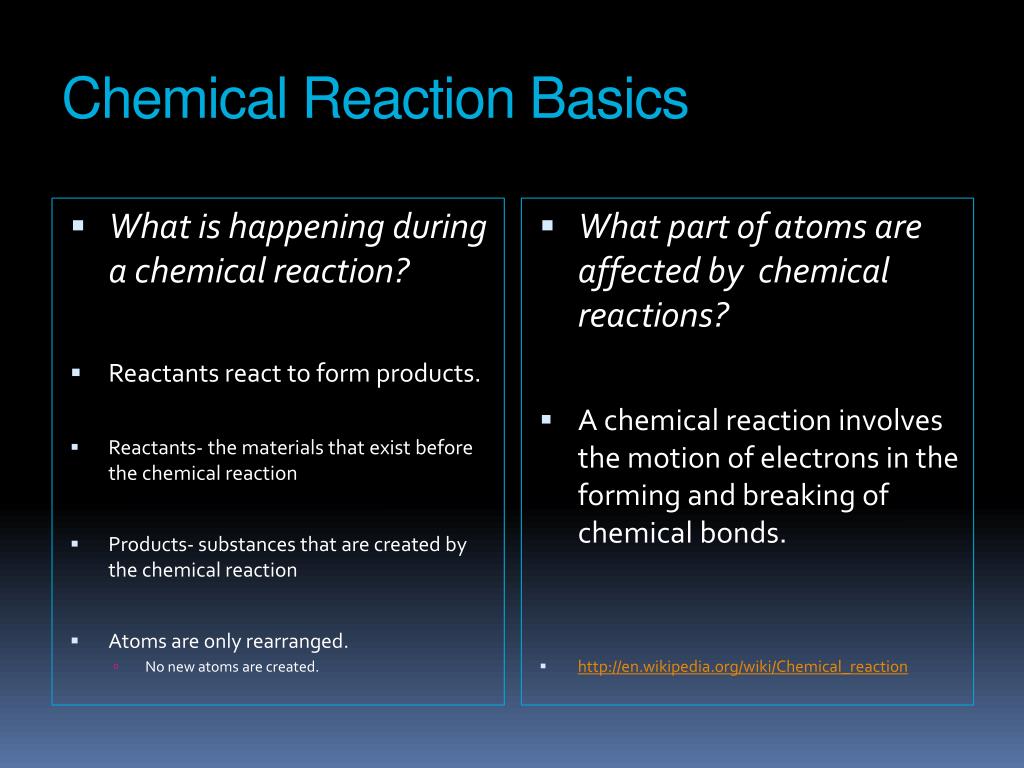
PPT Chemical Reactions (Chemical Rx) PowerPoint Presentation, free
Article 20 December 2023 | Open Access Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation Cysteine bioconjugation is an important method to modify biomolecules, but.
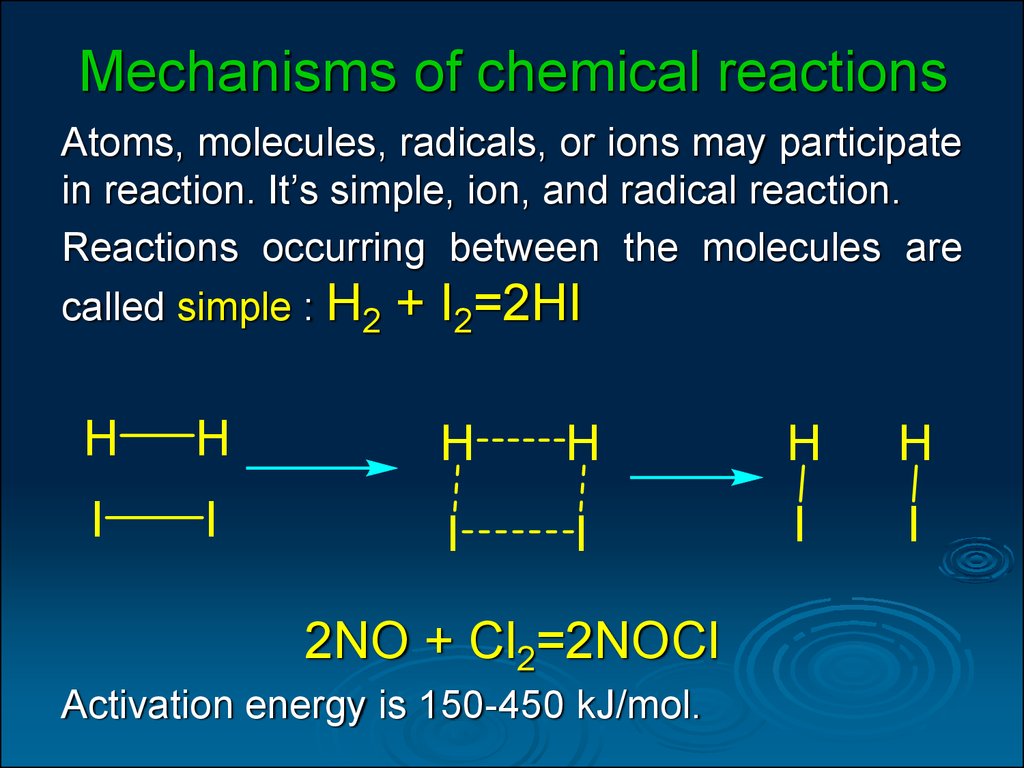
Factors affecting the rate of chemical reaction online presentation
Chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds.. Chain reactions and photolysis reactions are examples of classification by reaction mechanism, which provides details on how atoms are shuffled.

The reaction mechanism of sulfuric acid with polyethylene, introducing
The reaction mechanism (or reaction path) provides details regarding the precise, step-by-step process by which a reaction occurs. The decomposition of ozone, for example, appears to follow a mechanism with two steps: O3(g) O2(g) + O O +O3(g) 2O2(g) O 3 ( g) O 2 ( g) + O O + O 3 ( g) 2 O 2 ( g)
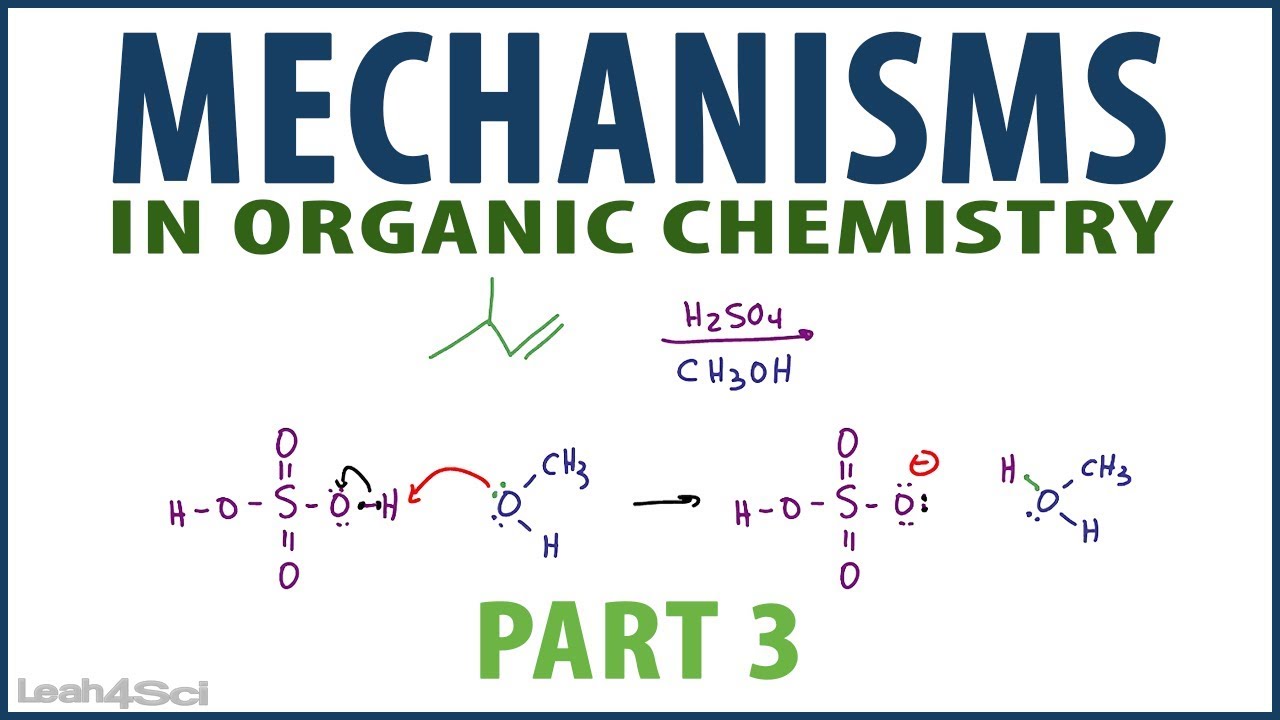
Organic Chemistry Reaction Mechanism Pattern Examples YouTube
A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. Many reaction mechanisms contain one step that is much slower than the others; this step is known as the rate-determining step. If the rate-determining step is the first step in a mechanism, the rate law for the overall reaction can be derived directly.
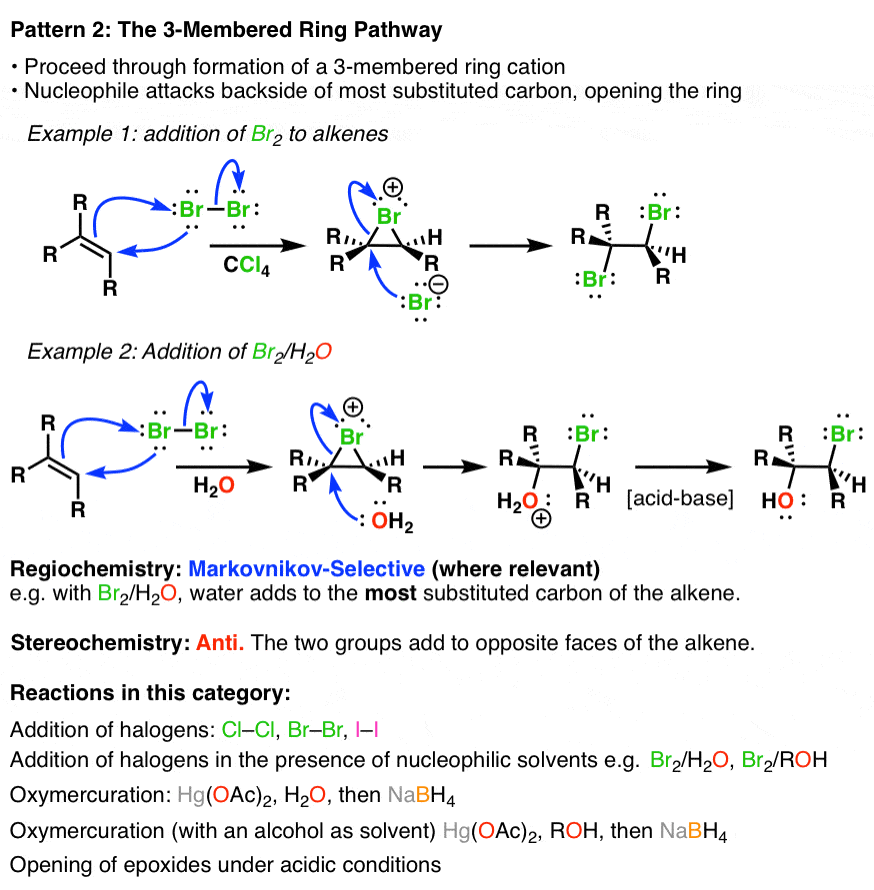
Summary Alkene Reaction Pathways — Master Organic Chemistry
The reaction mechanism (or reaction path) is the process, or pathway, by which a reaction occurs. A chemical reaction usually occurs in steps, although it may not always be obvious to an observer. The decomposition of ozone, for example, appears to follow a mechanism with two steps:

The basic chemical reaction mechanisms of glycosylation. (a) Reaction
However, some unimolecular reactions may be the only step of a single-step reaction mechanism. (In other words, an "overall" reaction may also be an elementary reaction in some cases.) For example, the gas-phase decomposition of cyclobutane, C 4 H 8 , to ethylene, C 2 H 4 , is represented by the following chemical equation:

chemistry world E1 REACTION MECHANISM & EXAMPLES
A reaction mechanism is the sequence of elementary steps by which a chemical reaction occurs. A reaction that occurs in two or more elementary steps is called a multistep or complex reaction. A reaction intermediate is a chemical species that is formed in one elementary step and consumed in a subsequent step.
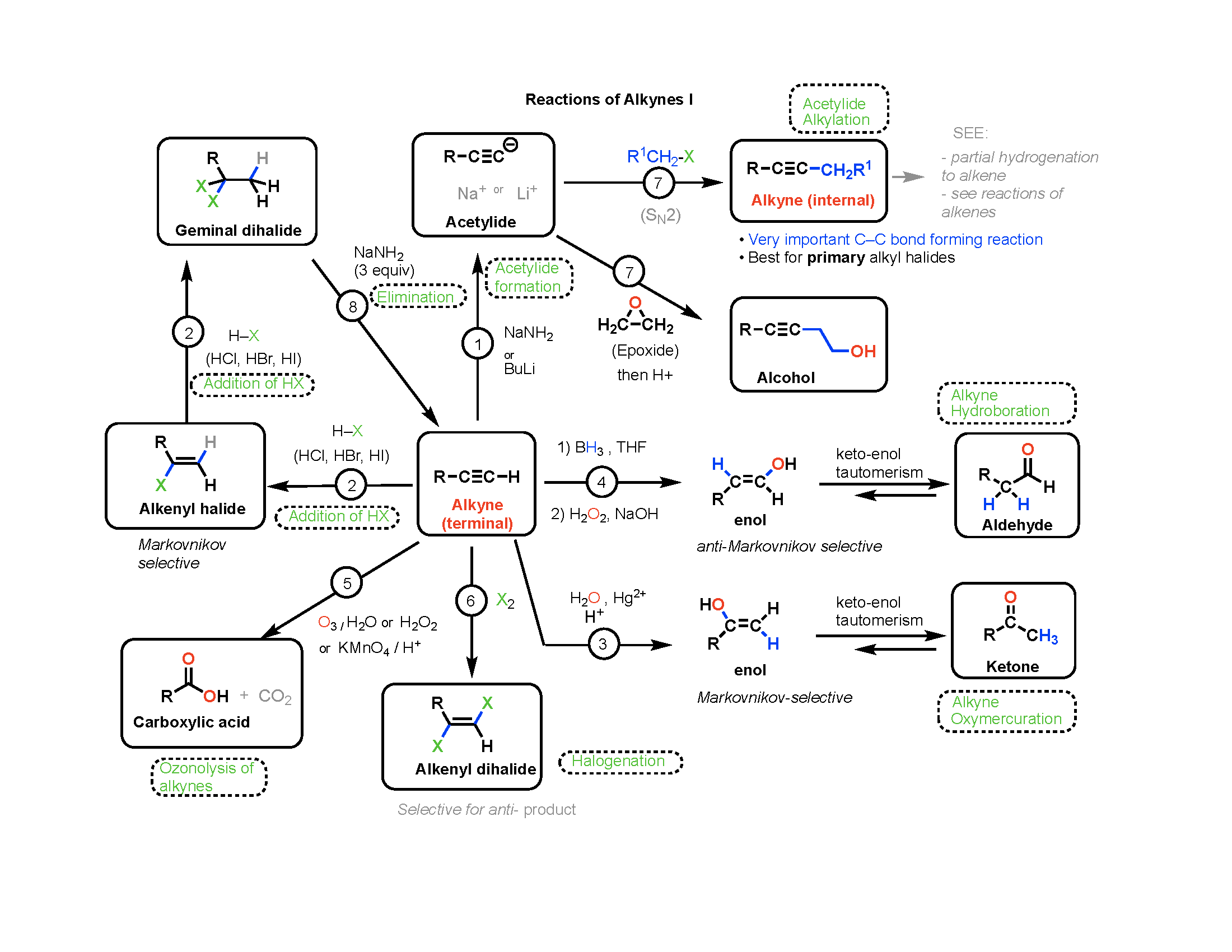
Reaction Maps Now Available Master Organic Chemistry
reaction mechanism, in chemical reactions, the detailed processes by which chemical substances are transformed into other substances. The reactions themselves may involve the interactions of atoms, molecules, ions, electrons, and free radicals, and they may take place in gases, liquids, or solids —or at interfaces between any of these.
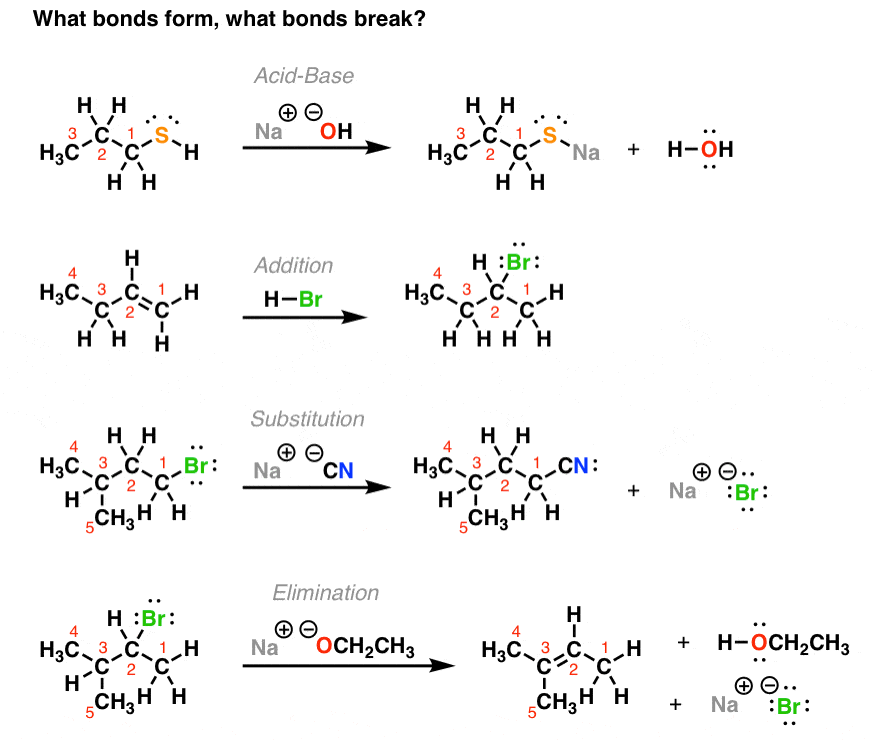
The 4 Major Classes of Reactions in Org 1 Master Organic Chemistry
Chemical reactions, Kinetic analysis, Order, Reaction mechanisms, Students Abstract An introductory guide to deducing the mechanism of chemical reactions is presented. Following a typical workflow for probing reaction mechanism, the guide introduces a wide range of kinetic and mechanistic tools.

Types of Chemical Reactions Chemical reactions, Chemistry basics
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. [1] A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases.
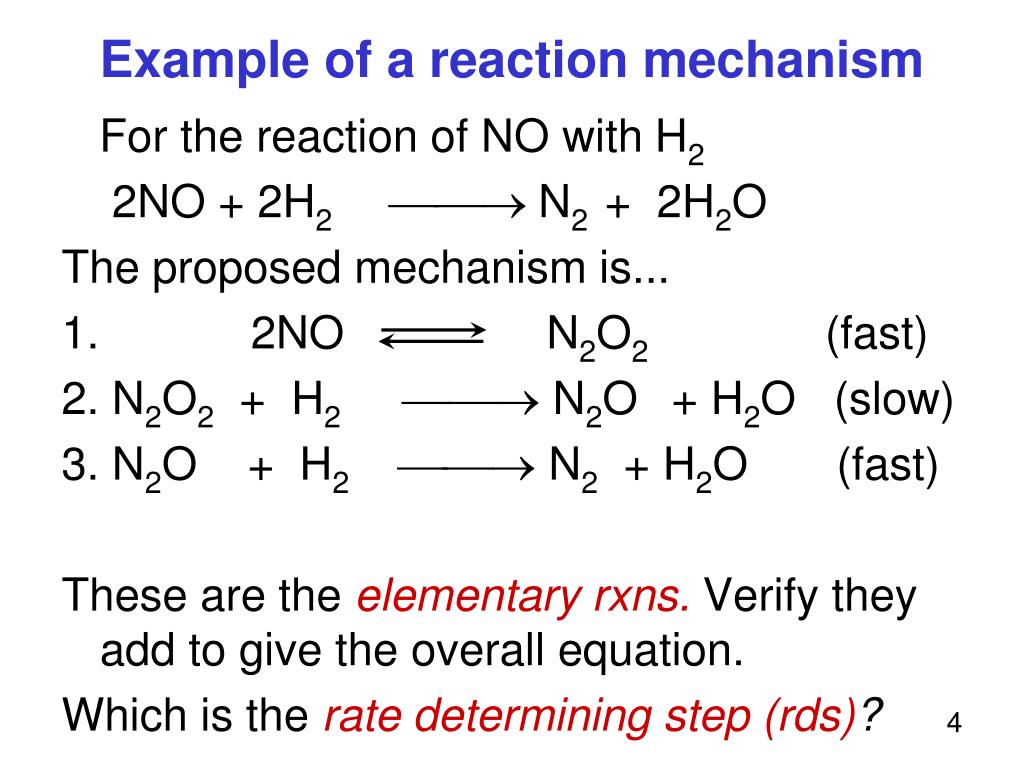
PPT Part V Reaction Mechanisms PowerPoint Presentation
For instance, Lowry and Richardson say: "A reaction mechanism is a specification, by means of a sequence of elementary chemical steps, of the detailed process by which a chemical change occurs" [Lowry and Richardson, 1987, p. 190]. These two features of mechanisms are related and both are important to the role of mechanisms in understanding.